Abstract
Sumário
Much cuttlefish research during the last few years has focused on its introduction as a new species for aquaculture. This is because the species displays biological and economical aspects with potential for industrial culture. Sykes et al. (2006a) reviewed this potential and identified reproduction as a technological bottleneck for the European cuttlefish, Sepia officinalis. This was mostly due to several biological aspects of the species, such as: semelparity; low fecundity and fertility in captive conditions; and suspicions of inbreeding (after egg non-viability after 6 consecutive generations - Sykes et al. 2006b). Control of reproductive function in captivity is essential for the sustainability of commercial aquaculture production. It relies on species specific biological and physiological knowledge and culture conditions, which will ultimately influence animal welfare (Conte 2004). Nonetheless, no previous studies on cuttlefish reproduction in captivity have used a multidisciplinary approach to solve these problems. With the present proposal we aim at an approach that will take zoo-technology, behavior, physiology, and population genetics, into account.
Regarding the effect of culture conditions, early experiments (Forsythe et al. 1991 and 1994; Correia et al. 2005) and recent data (Sykes et al. 2006a & 2006b, 2009 and unpublished results; Domingues & Márquez 2010) indicate that the type of tank, environmental and biological conditions may influence fecundity and fertility. On the other hand, the effects of optimal bottom area/tank volume, density, and natural male/female ratio; are still to be unveiled and these culture conditions will have an effect on cuttlefish physiology.
Knowledge regarding cuttlefish behaviour and chemical communication has been reported both in nature and captivity. In nature, cuttlefish only become social for reproduction, while in captivity they show complex intraspecific visual displays (Hanlon et al. 1999) and form short-term female-male pair associations (Boal, 1997). Males use visual displays to establish size-based dominance hierarchies, where large males mate more frequently (Adamo & Hanlon, 1996; Boal, 1997). During copulation, males display sperm removal behaviour (Hanlon et al. 1999). Despite the wealth of information regarding the role of vision in cuttlefish behaviour, little is known about the role(s) of olfaction. Apparently, olfaction is involved both in female mate-choice and social recognition (Boal & Golden, 1999; Boal, 2006). Recently, a peptide (ILME) was identified in cuttlefish, which is a chemical messenger released by the oocytes and eggs and which acts at both paracrine and pheromonal levels (Zatilny et al. 2000a) So, to what extent does all of this influence reproduction in captivity and how can we use it to manipulate cuttlefish reproduction? On the other hand, to what extent will culture conditions influence cuttlefish behavior and chemical communication?
If we are able to succeed in raising reproduction numbers in captivity to what is recorded in nature, then the future cuttlefish aquaculture industry will have to rely on a breeding selection protocol that still needs development. S. officinalis is a semelparous species and this implies a different brood stock management that used for most finfish. Until now, it has been a common practice to use cultured broodstocks to obtain animals for the subsequent generations (Sykes et al. 2006b). Such closed-cycle practice with captive breeders may have led to reproductive isolation from wild populations and a resultant loss of genetic variability due to the low effective breeding population size and inbreeding. We need to address this issue, by determining the effective number of breeders contributing for reproduction in an integrative way, by using behavioral analysis and paternity studies, and quantifying the loss of genetic variation in consecutive cultured generations at given culture conditions. To achieve this objective, we will have two lines of breeders: one according to Sykes et al. (2006a) and another were we will establish the minimum level of outbreeding by adding “new blood” at different generations.
After, we will use the data to redesign the existing cuttlefish husbandry protocol based on a statistical approach that will determine the importance of each variable under study in this project and the magnitude of influence in cuttlefish reproduction in captivity.
The accomplishment of the objectives of the current proposal will allow not only a better understanding of cuttlefish biology, behaviour and genetics, but will also be of extreme importance for other cephalopod species and industry application in the future.
Uma parte considerável da investigação no choco durante os últimos anos tem-se centrado na sua introdução como uma nova espécie em aquacultura. Tal deve-se à mesma apresentar aspectos biológicos e económicos com potencial para aplicação industrial. Sykes et al. (2006a) reviu este potencial e identificou a reprodução como um “bottleneck” tecnológico para o choco Europeu, Sepia officinalis. Tal deveu-se a vários aspectos biológicos da espécie, tais como: semelparidade; fecundidade e fertilidade em cativeiro; e suspeitas de endogamia (falta de fertilidade dos ovos após 6 gerações consecutivas - Sykes et al. 2006b). O controlo da reprodução em cativeiro é essencial para a sustentabilidade da produção em aquacultura comercial e depende no conhecimento biológico e fisiológico característico da espécie e das condições de cultivo, que em último caso influenciam o bem-estar animal (Conte 2004). No entanto, nenhum estudo prévio da reprodução do choco em cativeiro realizou uma abordagem multidisciplinar na solução destes problemas. A presente proposta visa uma abordagem que irá tomar em conta a zoo-tecnologia, comportamento, fisiologia e genética populacional.
Em relação ao efeito das condições de cultivo, ensaios anteriores (Forsythe et al. 1991 e 1994; Correia et al. 2005) e dados recentes (Sykes et al. 2006a & 2006b, 2009 e resultados não publicados; Domingues & Márquez 2010), indicam que o tipo de tanque, as condições ambientais e biológicas podem influenciar a fecundidade e fertilidade. Por outro lado, os efeitos da área de fundo/volume do tanque, densidade e rácio natural de macho/fêmea; ainda são desconhecidos e estas condições de cultivo terão um efeito na fisiologia do choco.
Conhecimento acerca do comportamento e de comunicação química no choco têm sido descritos na natureza e em cativeiro. Na natureza, os chocos são apenas sociais durante a reprodução, enquanto em cativeiro, exibem imagens intra-específicas complexas (Hanlon et al. 1999) e formam casais de curto prazo (Boal, 1997). Os machos usam estas imagens para estabelecer hierarquias de dominância, através das quais os maiores copulam mais frequentemente (Adamo & Hanlon, 1996; Boal, 1997). Durante a cópula, os machos exibem ainda um comportamento de remoção do esperma (Hanlon e tal. 1999). Apesar da variedade de informação disponível no que concerne à função da visão no comportamento do choco, pouco se conhece no que respeita à função do olfacto. Sabe-se apenas que o choco usa o olfacto para detecção de sinais ambientais, escolha de parceiros e reconhecimento social (Boal & Golden, 1999; Boal, 2006). Recentemente, um péptido foi identificado na espécie, com funções que parecem ser as de um mensageiro químico libertado pelos oócitos e ovos, capaz de exercer actividade parácrina e feromonal (Zatilny et al. 2000a). Mas até que ponto todos estes factores influenciam a reprodução em cativeiro do choco e como podemos utilizar este conhecimento na manipulação da mesma? Por outro lado, até que ponto o comportamento e a comunicação química é influenciada pelas condições de cultivo?
Se formos bem sucedidos no incremento dos valores de reprodução em cativeiro em relação ao que está descrito para o meio natural, então a futura indústria de cultivo do choco terá que depender num protocolo de gestão de reprodutores que necessita ser desenvolvido. O choco é uma espécie semelpátrica e este factor implica uma gestão do stock de reprodutores distinta da adoptada para a maioria das espécies de peixe. Até agora tem-se utilizado reprodutores cultivados para a obtenção das gerações consecutivas (Sykes et al. 2006b). Esta prática poderá ter originado isolamento populacional e redução da variabilidade genética, devido à pequena população de reprodutores utilizados em cada geração, e efeitos de endogamia. Este problema deve ser abordado através da determinação do número efectivo de reprodutores que contribuem para a geração seguinte, de um modo integrado e que inclua análise comportamental, estudos de paternidade e quantificação de variação genética; em determinadas condições de cultivo. Para atingir este objectivo iremos estudar duas linhas de reprodutores: uma cultivada de acordo com Sykes et al. (2006a) e outra onde poderemos determinar o nível mínimo de outbreeding através da introdução de “sangue novo” em gerações distintas.
De seguida, iremos utilizar os dados para redesenhar o protocolo de reprodução existente baseando-nos numa abordagem estatística que irá determinar a importância e a magnitude de influência de cada variável em estudo no projecto na reprodução do choco em cativeiro.
O bom concretizar dos objectivos propostos irá permitir não só uma melhoria do conhecimento da espécie ao nível da biologia, comportamento e genética, mas será ainda de importância relevante para outras espécies de cefalópodes e numa aplicação industrial futura.
Research Plan and Methods
The reproduction of the European cuttlefish, S. officinalis, in captivity is currently a bottleneck in the development of the species culture (Sykes et al. 2006a). This is mainly due to scattered information and the use of different methodologies between culture laboratories, which derive from different objectives for its culture. Cuttlefish is cultured as animal model for biomedical research (e.g. neuroscience), for aquaculture production and for aquariums/public exhibition. Recently, some of the species characteristics also point to an eventual use in cancer research. On the other hand, its culture may also contribute to biodiversity and conservation of wild stocks.
This proposal aims to increase the fecundity and fertility; in determining the existence of genetic erosion and also to solve this problem. The innovative challenge in the current proposal is to understand the physiology, behavior and population genetics interfering with reproduction of cuttlefish stocks under different culture conditions and in a multidisciplinary way. This is particularly important as most of the work on cuttlefish reproduction in captivity did not take these aspects into account nor used this integrative approach.
Considering all of these key aspects, we gathered a workgroup with CCMAR researchers who combine expertise in biology, zoo-technology, population genetics, animal physiology and behavior, to work together in the use of a multidisciplinary approach on the subject. Moreover, we invited 3 consultants to this proposal to enrich the discussion and feedback during the project.
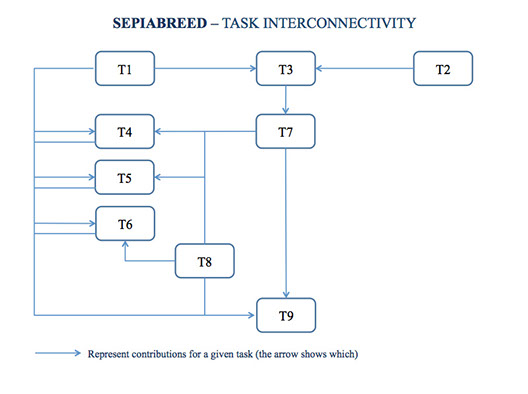
Due to the nature of these objectives, to the several fields of research involved, and to the gathering of the required data, the several tasks are interconnected (see DIAGRAM) and/or will run at the same time and reflect the integrative approach to the identified problems. To do so, we will first culture 2 consecutive generations in triplicate (T1), which will be reared at different bottom areas/tank volumes to perceive whether this different culture conditions will have an effect on the species growth and reproduction (M2) as well as behavior, chemical communication, paternity and rate of inbreeding. Regarding the first, data will be collected according to variables already assessed by Sykes et al. (2006b) in a recent consecutive generation study. In addition, animals will be filmed; water samples and tissue samples will be collected for the assessment of the latter.
At the same time, we will characterize the basic anatomy and physiology of the olfactory system of cuttlefish to obtain technology and a methodology (T2 and M1) that will allow us to understand the importance of chemical communication in reproduction (T3). This will be done by following a similar methodology to that of Mobley et al. (2008), which include the use of histology techniques to identify receptor neurons or other types of cells in the olfactory system and the organization of the olfactory bulb. Then, olfactory responses to known odorants, such as amino acids described for squid, will be investigated by extracellular recording from the olfactory nerve or bulb. This recording will be performed after adaptation of one of the methods described by Hubbard et al. (2003), Hubbard & Canário (2007) or Serrano et al. (2008). Water samples obtained from immature and mature male and females of the different bottom areas/tank volumes experiment will then be processed by fraction and according to a solid-phase extraction (SPE) protocol adapted from Huertas et al. (2007). The olfactory sensitivity of cuttlefish to these fractions, samples of body fluids (urine and faeces), and to extracts of cuttlefish mature oocytes (Zatylny et al. 2002) and fertilized eggs (Zatylny et al. 2000a) will then be assessed by electrophysiological recording. Composition of the odorants that give higher responses will be determined by purifying them using HPLC (similar to that described by Serrano et al. 2008 and Velez et al. 2009), and putatively identified (M3) by liquid chromatography-mass spectroscopy or gas chromatography-mass spectroscopy, as appropriate (e.g. Velez et al. 2009). We will then test the use of identified putative pheromones by performing an experiment where 2 cuttlefish stocks in triplicate will be used. One will be manipulated by adding the odorants with higher response to water containing immature cuttlefish and by determining if it promotes precocious gonadal development (T7 and M7), while the other will act as control. If reproduction is achieved by manipulation, estimates of growth and reproduction data (similar to that described in T1) will be assessed and compared with those of T1. In addition, paternity (T5) and behavior (T4) will also be studied and compared with control and other tasks.
Still regarding the rearing at different bottom areas/tank volumes (T1), the reproductive and social behavior under these culture conditions will be determined with the aid of underwater video cameras linked to digital video recording devices. Digital videos will be analyzed for behavior using the computerized system, “The Observer”, as described in Barata et al. (2008), to assess whether and how different culture conditions affect the reproductive and social behavior of the species (T4), which include the eventual establishment of mating hierarchies and the existence of sneaker males (M4). On the other hand, the use of wild animals as part of the systematic line crossing (T8) and the use of pheromones (T7) might also originate different behaviors and hierarchies within the culture tanks. Therefore, specific behavior resulting from the introduction of wild cuttlefish as part of systematic line crossing (T8) and the presence of a given odorant/pheromone as maturation and spawning promoter (T7) will also be filmed and processed using “The Observer”.
Paternity analysis (T5) and the rate of inbreeding (T6) will be studied when rearing at different bottom areas/tank volumes (T1), using an odorant/pheromone as promoter of reproduction (T7) and the effect of systematic line crossing (T8). Genetic erosion and paternity analysis will be assessed by microsatellite analysis of the 7 microsatellite loci previously isolated and characterized for the species by Shaw & Pérez-Losada (2000). PCR amplification and genotyping of individuals will follow the methods of Shaw & Pérez-Losada (2000). Unbiased expected heterozygosity will be calculated using GENEPOP software (Raymond & Rousset, 1995). Individual inbreeding model (Chybicki & Burczyk, 2009), implemented in the software INEST, will be used to calculate null allele frequencies and the average level of inbreeding. Regarding paternity, we will look for mismatches between parents and offspring using the matrix outputs from the software CERVUS 3.0 (Kalinowski et al. 2007). Relatedness coefficients between all possible pairs of breeders will be obtained with the r estimator (Wang 2002) using the statistical package Kingroup2 (Konovalov et al. 2004). Genetic diversity estimators and the two exclusion probabilities (Excl1, Excl2) for each locus and for all loci, will be calculated using the allele frequency option of CERVUS 3.0. Deviations from HW equilibrium and linkage disequilibrium between all pairs of loci will be tested using the GENEPOP 3.1 package, (Raymond & Rousset 1995). An estimation of null allele frequency at each locus will be obtained using genotype data of broodstock individuals with CERVUS 3.0.
Finally, the existence of inbreeding and the effects of systematic line crossing (T8) in the reduction of the rate of inbreeding accumulation will be assessed by culturing 2 stocks of cuttlefish in triplicate for 5 consecutive generations. The use of systematic line crossing schemes to eliminate the mating of close relatives is one of the breeding approaches to avoid or reduce inbreeding (Fjalestad, 2005). In one of the stocks, every 2 generations and as sexual maturity is reached, triplicates of 6 females will be selected and placed in three separated tanks, 2 females in each tank. A mature male caught from the wild will be placed in each tank for mating. Hatchlings resulting from the mixing of cultured and wild cuttlefish will be mixed with offspring resulting from the normal consecutive generation stock (which result from 2 or more generations in captivity), resulting in a new broodstock. This new broodstock will then be cultured in a similar way to that of captive consecutive generations for the following 2 generations, when mixing of cultured and wild animals will be performed once again. These stocks will be screened for paternal contribution (T5) and rate of inbreeding (T6). The determination of parental contribution at given culture conditions, the extent of inbreeding and of genetic pool renewal of consecutive cultured cuttlefish generations are also considered milestones (M6).
All of these data will then be considered in the elaboration of a first management and selective breeding protocol for cuttlefish (T9) by principle component analysis, linear discriminant analysis and logistic regression (Manly, 1994); which will be used to determine the magnitude of correlations involved in obtaining eggs in quantity and quality. Consultants will provide an outside view of the project’s results for every task which will enrich the argument of the themes discussed.
At the end of this project, we expect to have achieved a clarification on the role and magnitude of each studied variable on cuttlefish reproduction. We believe that the results of this project will allow us to obtain a similar reproduction potential in captivity as seen in the wild and mitigate the problem of inbreeding. The basic and applied research results obtained here might also have application in other cephalopod aquaculture candidates and will certainly contribute for the establishment of culture welfare practices and conservation of the wild stocks of the species.
Bibliography
Adamo, S.A. & Hanlon, R.T. (1996). Do cuttlefish (Cephalopoda) signal their intentions to conspecifics during agonistic encounters? Animal Behaviour 52, 73-81.
Barata, E.N., Serrano, R.M., Miranda, A., Nogueira, R., Hubbard, P. C. & Canário, A.V.M. (2008). Putative pheromones from the anal glands of male blennies attract females and enhance male reproductive success. Animal Behaviour 75, 379-389.
Boal, J.G. (1997) Female choice of males in cuttlefish (Mollusca: Cephalopoda). Behaviour 134, 975-988.
Boal, J.G. (2006) Social recognition: A top down view of cephalopod behaviour. Vie et Milieu-Life and Environment 56, 69-79.
Boal, J.G. & Golden, D.K. (1999) Distance chemoreception in the common cuttlefish, Sepia officinalis (Mollusca, Cephalopoda). Journal of Experimental Marine Biology and Ecology 235, 307-317.
Chybicki, I.J. & Burczyk, J. (2009). Simultaneous estimation of null alleles and inbreeding coefficients. Journal of Heredity 100 (1), 106-113.
Conte, F.S. (2004). Stress and the welfare of cultured fish. Applied Animal Behaviour Science 86, 205-223.
Correia, M., Domingues, P.M., Sykes, A. & Andrade, J.P. (2005). Effects of culture density on growth and broodstock management of the cuttlefish, Sepia officinalis (Linnaeus, 1758). Aquaculture 245, 163-173.
Domingues, P. & Márquez, L. (2010). Effects of culture density and bottom área on growth and survival of the cuttlefish Sepia officinalis (Linnaeus, 1758). Aquaculture International 18, 361-369.
Fjalestad, K.T. (2005). Breeding strategies. In: Selection and breeding programes in Aquaculture (Gjedren, T., editor): 145-158. Dordrecht Springer, Amsterdam.
Forsythe, J.W., DeRusha, R.H. & Hanlon, R.T. (1994). Growth, reproduction and life span of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. J. Zool. London 233, 175-192.
Forsythe, J.W., Hanlon, R.T., & DeRusha, R.H. (1991). Pilot large-scale culture of Sepia in biomedical research. In: La seiche (E. Boucaud-Camou ed.), pp. 313-323. Acta of the First International Symposium on the Cuttlefish Sepia officinalis. Centre de Publications de l’Université de Caen.
Hanlon, R.T., Ament, S.A. & Gabr, H. (1999). Behavioral aspects of sperm competition in cuttlefish, Sepia officinalis (Sepioidea: Cephalopoda). Marine Biology 134, 719-728.
Hubbard, P.C., Barata, E.N. & Canário, A.V.M. (2003). Olfactory sensitivity of the gilthead seabream (Sparus auratus, L.) to conspecific body fluids. Journal of Chemical Ecology 29 (11), 2481-2498.
Hubbard, P.C. & Canário, A.V.M. (2007). Evidence that olfactory sensitivities to calcium and sodium are mediated by different mechanisms in the goldfish Carassius auratus. Neuroscience Letters 414, 90-93.
Kalinowski, S.T., Taper, M.L. & Marshall, T.C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology 16, 1099-1106.
Konovalov, D.A., Manning, C. & Henshaw, M.T. (2004). KINGROUP: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Molecular Ecology Notes 4, 779-782.
Manly, B.F.J. (1994). Multivariate Statistical Methods: a primer. Chapman & Hall, London. 159p
Mobley, A.S., Michel, W.C. & Lucero, M.T. (2008). Odorant responsiveness of squid olfactory receptor neurons. The Anatomical Record 291, 763-774.
Raymond, M. & Rousset, F. (1995). GENEPOP (Version 1.2): Population genetics software for exact tests and ecumenicism. Journal of Heredity 86 (3), 248-249.
Serrano, R.M., Barata, E.N., Birkett, M.A., Hubbard, P.C., Guerreiro, P.S., Canário, A.V.M. (2008) Behavioral and Olfactory Responses of Female Salaria pavo (Pisces: Blenniidae) to a Putative Multi-component Male Pheromone. Journal of Chemical Ecology 34, 647-658.
Shaw, P.W. & Pérez-Losada, M. (2000). Polymorphic microsatellites in the common cuttlefish Sepia officinalis (Cephalopoda). Molecular Ecology 9, 237-244.
Sykes, A.V., Domingues, P.M., Correia, M., & Andrade, J.P. (2006a). Cuttlefish culture – state of the art and future trends. Vie et Milieu 56(2), 129-137.
Sykes, A.V., Domingues, P.M. & Andrade, J.P. (2006b). Effects of using live grass shrimp (Palaemonetes varians) as the only source of food for the culture of cuttlefish, Sepia officinalis (Linnaeus, 1758). Aquaculture International 14(6), 551-568.
Sykes, A.V., Almansa, E., Lorenzo, A. & Andrade, J.P. (2009). Lipid characterization of both wild and cultured eggs of cuttlefish (Sepia officinalis) throughout the embryonic development. Aquaculture Nutrition 15, 38-53.
Velez, Z., Hubbard, P., Welham, K., Hardege, J.D., Barata, E.N. & Canario, A.V.M. (2009) Identification, release and olfactory detection of bile salts in the intestinal fluid of the Senegalese sole (Solea senegalensis). Journal of Comparative Physiology 195, 691-698.
Wang, J. (2002). An estimator for pairwise relatedness using molecular markers. Genetics 160, 1203-1215.
Zatylny, C., Gagnon, J., Boucaud-Camou, E. & Henry, J. (2000a). ILME: A waterborne pheromonal peptide released by the eggs of Sepia officinalis. Biochemical and Biophysical Research Communications 275, 217-222.
Zatylny, C., Marvin, L., Gagnon, J. & Henry, J. (2002). Fertilization in Sepia officinalis: the first mollusk sperm-attracting peptide. Biochemical and Biophysical Research Communications 296, 1186-1193.
GOOGLE EARTH
CONNECT WITH US
NEWSLETTER